On November 1, the Centers for Medicare & Medicaid Services (CMS) released the calendar year 2025 (CY2025) Final Rule for the Medicare Physician Fee Schedule (MPFS), which also includes proposals related to the Quality Payment Program (QPP). The following AAO-HNS overview provides details regarding the rule’s key provisions impacting the field of otolaryngology.
Physician Fee Schedule:
A. Proposed CY 2025 Conversion Factor: The final rule sets the MPFS conversion factor at $32.35, a decrease of $0.94 (2.83%) from the CY 2024 conversion factor of $33.29.
B. Estimated Impact to Otolaryngology: CMS estimates the overall impact of the MPFS final changes for 2025 to be 0% for otolaryngology. It is important to note that this does not include the 2.83% cut all clinicians are subject to.
C. HCPCS Code G2211: The rule finalizes the payment allowance for the G2211 add-on code when reported by the same practitioner on the same day as an annual wellness visit, vaccine visit, or any Medicare Part B preventative service.
D. Global Surgical Period Accuracy: The rule finalizes a policy to broaden the applicability of the transfer of care modifier -54 for all 90-day global surgical packages in cases where the provider plans to only furnish the surgical procedure itself. The final rule requires modifier -54 to be appended to the initial procedure when the practitioner only intends to perform the procedure and does not intend to provide the post-operative care in both formal and informal scenarios.
E. New Surgical Code GPOC1: For 2025, CMS is finalizing an add-on code for post-operative care which is provided by a practitioner other than the one who performed the initial procedure (or another practitioner in their practice). Beginning January 1, 2025, add-on code G0559, post-operative follow-up visit complexity inherent to evaluation and management services addressing surgical procedure(s), provided by a physician or qualified health care professional who is not the practitioner who performed the procedure (or in the same group practice), will be active. CMS intends for this code to adequately reimburse practitioners who are providing the previously mentioned follow-up care for patients whom they are not familiar with. The RVU value for G0559 was finalized as 0.16 with a time of 5.5 minutes.
F. New Codes for Percutaneous Radiofrequency Ablation of Thyroid (CPT Code 60660 & 60661): This rule finalized the valuation of the percutaneous ablation of thyroid codes, 60660 and 60661 using the RUC recommended RVU and PE values. Code 60660, Ablation of 1 or more thyroid nodule(s), one lobe or the isthmus, percutaneous, including imaging guidance, radiofrequency, received a work RVU of 5.75. Code 60661, Ablation of 1 or more thyroid nodule(s), additional lobe, percutaneous, with imaging guidance, radiofrequency, received a work RVU of 4.25.
G. Telehealth Services under the MPFS:
i. Expiration of Originating Site and Geographic Waivers: Current flexibilities allow patients to receive telehealth services all over the country, not just in rural areas, and to receive these services in their homes without having to go to a medical facility. Without congressional action, these flexibilities will expire on December 31, 2024. After this date, Medicare telehealth services will generally be available only when the patient is in a medical setting in a rural area. An exception to this policy exists for patients receiving mental health and/or substance use disorder services.
ii. Discontinuation of Audiologists and Speech-Language Pathologists as Authorized Telehealth Providers: Beginning January 1, 2025, the statutory limitations that were in place for Medicare telehealth services prior to the COVID-19 Public Health Emergency (PHE) will once again take effect for most telehealth services. These include restrictions on where Medicare telehealth services can be provided (i.e., the expiration of some geographic and originating site waivers) and limitations on the scope of practitioners who can provide Medicare telehealth services. Absent Congressional action, this HHS policy change will discontinue the inclusion of audiologists and speech-language pathologists as authorized telehealth providers.
iii. Continued “Provisional” Status of Audiology and Speech-Language Pathology CPT Codes: In last year’s MPFS final rule, CMS created a 5-step process for adding, changing, or deleting any code on Medicare’s Telehealth Services List. Effective for CY2025, this new process removes the “Category 1” or “Category 2” categorizations for services and instead assigns each service a “permanent” or “provisional” status. In CY2024, several Audiology and Speech-Language Pathology Codes were assigned “provisional” status under the Medicare Telehealth Services List, which the Academy supported. Although the Academy strongly recommended that these services be recategorized as “permanent” for CY2025, CMS elected to maintain these codes with a provisional status on the CY2025 Medicare Telehealth Services List. CMS intends to conduct a comprehensive analysis of all such provisional codes, which is expected to be addressed in future rulemaking.
iv. Expansion of Audio-Only Coverage: Beginning January 1, 2025, CMS will allow telehealth providers to use audio-only communication in certain circumstances if the patient is not capable of, or does not consent to, the use of video technology. This is an expansion to previous CMS policy that allowed audio-only telehealth visits for mental health services. This rule will expand access to some Medicare telehealth services for patients who may not have the necessary technology or prefer not to use video for their appointments; however, the AAO-HNS opposes this policy, maintaining the position that audio-only services are not analogous to in-person care, nor are they an appropriate substitute for a face-to-face encounter. In the final rule, CMS clarifies that audio-only telehealth services will require no additional documentation, except for the addition of CPT modifier -93 or, for Rural Health Clinics or Federally Qualified Health Centers, the Medicare modifier “FQ.”
v. Revised Definition of “Direct Supervision”: For a certain subset of services that are required to be furnished under direct supervision, CMS is permanently adopting a definition of direct supervision that will allow such supervision to be provided virtually (through live video and audio telecommunication) by the physician or other supervising practitioner. Specifically, this permanent definition of direct supervision will apply to services performed “incident to” the physician’s services, when provided by auxiliary personnel. To qualify for reimbursement, these services must have an HCPCS code with a PC/TC indicator of “5” (usually used for diagnostic tests) or be billed under CPT code 99211 (E/M for a minimal office visit). Additionally, this permanent definition of direct supervision will apply to E/M services furnished to established patients who may not require the presence of a physician or other qualified health care professional. For all other services requiring direct supervision, CMS will continue to temporarily permit direct supervision via telecommunication through December 31, 2025. The Academy supports this policy, acknowledging that a permanent change in definition for a subset of services, and a broader change in definition only for the next year, strikes the right balance of ensuring access to high-quality care while mitigating program integrity concerns.
H. Valuation of Services:
i. Updates to Direct Practice Expense Inputs: CMS finalized its proposal, which was supported by the Academy, to update pricing for several direct practice expense inputs that affect Otolaryngology. Those include:
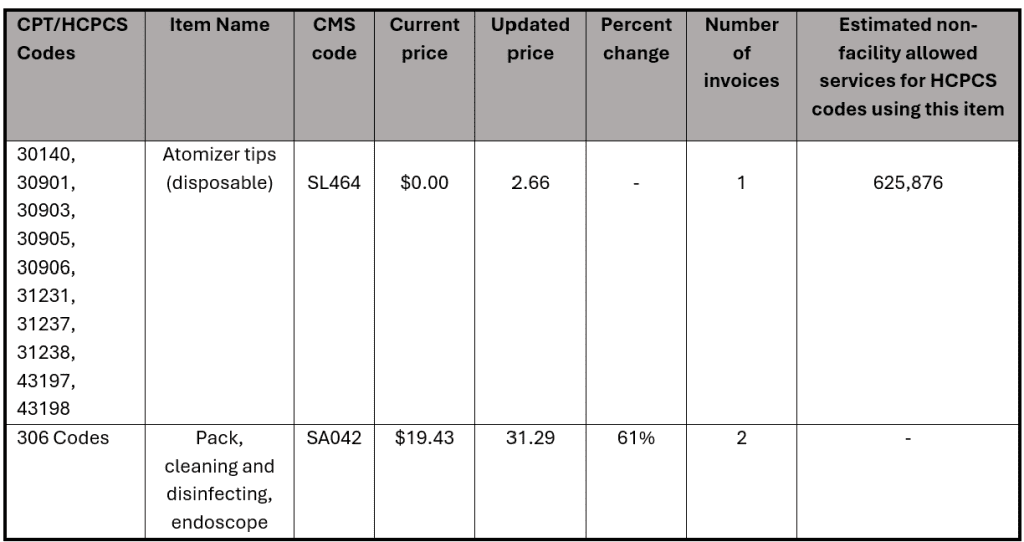
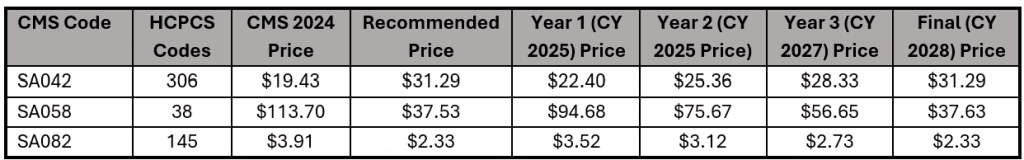
ii. Updates to Supply Pack Pricing: In the proposed rule, 18 codes related to supply packs were identified as incorrect. Upon review, CMS reported in the final rule that 15 of the supply packs referenced are included in the direct PE inputs for over 100 codes. For this reason, they are not finalizing a pricing update at this time. For the 3 remaining codes, CMS finalized a -year price transition to occur between 2025 and 2028. The chart below shows the finalized changes and schedule.
I. Potentially Misvalued Services Under the MPFS:
i. In-Office Tympanostomy Tubes: In the proposed rule, CMS requested comments regarding options to more accurately reimburse in-office tympanostomy tube placement, which is currently reimbursed using CPT code 69433. In the final rule, CMS acknowledged suggestions to better describe and reimburse the resources associated with innovative tympanostomy tube delivery using CPT code 0583T. However, CMS elected not to finalize national pricing for CPT code 0583T at this time. CMS also acknowledged suggestions to establish an add-on G-code with inputs based on the procedure being done in an office setting for patients at high risk of movement, such as pediatric patients. Most comments, including those submitted by the AAO-HNS, were in favor of the add-on G-code, which CMS elected to finalize as part of this rule. HCPCS code G0561, Tympanostomy with local or topical anesthesia and insertion of a ventilating tube when performed with tympanostomy tube delivery device, unilateral (list separately in addition to 69433), is to be billed in conjunction with CPT code 69433 to describe the additional resource costs associated with using the innovative delivery devices and/or systems which fall under emerging technology. This new add-on code should not be used in conjunction with code 0583T.
ii. Unattended Sleep Study (CPT Code 95800): In the proposed rule, CMS requested comments on whether CPT code 95800, Sleep study, unattended, simultaneous recording; heart rate, oxygen saturation, respiratory analysis (e.g., by airflow or peripheral arterial tone), and sleep time, should be included on the misvalued code list. The nominator suggested that the code be included based on an alleged shift towards disposable devices and away from reusable devices. Based on a lack of sufficient evidence or clear consensus as to which is more common, CMS finalized its proposal to not include this code on the misvalued list. CMS intends to reassess when more information is made available.
iii. Fine Needle Aspiration Codes (CPT Codes 10004, 10005, 10006, & 10021): In the proposed rule, the CPT codes for fine needle aspiration were nominated as potentially misvalued. The Academy submitted comments addressing this concern; AAO-HNS does not agree that the codes are currently misvalued and does not support resurvey of the codes at this time. In the final rule, CMS elected to not include these codes as potentially misvalued, noting that the “valuation accurately reflects the typical work and direct PE inputs involved in furnishing FNA services.”
Quality Payment Program
A. Scoring and Data Completeness
i. Performance Threshold: In the final rule, CMS finalized its decision to maintain the current performance threshold policies, meaning the performance threshold will remain at 75 points for the 2025 calendar year/performance period, or the 2027 MIPS payment year.
ii. Flat Benchmarking: CMS did not respond to the suggestion to expand flat benchmarking to other quality measure types beyond the Medicare CQM collection type for ACOs.
iii. Reweighting: CMS finalized the proposal to allow clinicians to request reweighting for quality, improvement activities, and/or Promoting Interoperability performance category (or categories) where data are inaccessible and unable to be submitted due to reasons outside of the control of the clinician because the clinician delegated submission of the data to their third party intermediary (evidenced by a written agreement) and the third party intermediary didn’t submit the data on the clinician’s behalf in accordance with applicable deadlines. However, there was no response to the suggestion to expand on the qualifications when a practice changes EHRs mid-year or when an EHR is unwilling to share data to facilitate MIPS reporting.
B. Quality Performance Category
i. This rule finalized five new quality measures, including
1) Q506: Positive PDL1 Biomarker Expression Test Result Prior to First Line Immune Checkpoint Inhibitor Therapy
2) Q507: Appropriate Germline Testing for Ovarian Cancer Patients
3) Q508: Adult COVID19 Vaccination Status
4) Q509: Melanoma: Tracking and Evaluation of Recurrence
5) Q510: First Year Standardized Waitlist Ratio (FYSWR)
C. Improvement Activity Performance Category
i. Removal of Improvement Activity Weights: The final rule removes improvement activity weighting and streamlines the reporting requirements for the performance category.
ii. IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time Access to Patient’s Medical Record: The final rule also removes IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time Access to Patient’s Medical Record from use beginning in January 2025.
D. Cost Performance Category
i. Cost Measure Inventory: CMS updated the cost measure inventory to include 6 episode-based cost measures related to acute inpatient respiratory infection, chronic kidney disease, end-stage renal disease, kidney transplant management, prostate cancer, and rheumatoid arthritis.
E. MIPS Value Pathways
i. Traditional MIPS transition to MVP: CMS has not communicated a final date to fully transition away from traditional MIPS and require MVP reporting. CMS finalized six new MVPs that will be available beginning with the 2025 performance period related to ophthalmology, dermatology, gastroenterology, pulmonology, urology, and surgical care.
ii. MVP Support Requirements: The final rule changes the support requirements for MVPs. Participants will no longer be required to select a population health measure as part of their MVP registration. One will be assigned if it is available and appropriate.
iii. ENT MVP Quality Measures: The Quality Care for the Treatment of Ear, Nose, and Throat Disorders MVP was revised to remove two of the twelve quality measures:
1) Quality ID: AAO16 Age-Related Hearing Loss: Comprehensive Audiometric Evaluation
2) Quality ID: AAO23 Allergic Rhinitis: Intranasal Corticosteroids or Oral Antihistamines
F. Third-Party Intermediaries: CMS finalized the elimination of the health IT vendor category of third-party intermediaries, beginning with the 2025 performance period, to remove gaps in third-party intermediary requirements and improve data integrity. To submit data on behalf of clinicians, a health IT vendor (i.e., EHRs) will need to meet specific requirements and self-nominate to become a qualified registry or QCDR.
